“We should move forward with this.” Six simple words in an email that might have just legally committed your company to a million-dollar deal. Surprised? You’re not alone. The line between casual business conversations and legally binding commitments is often much thinner than most professionals realize.
Think about your last week at work. How many times did you exchange emails about potential deals? Did you nod in agreement during a video call about next steps? Maybe you gave verbal approval to a supplier’s proposal? Any of these scenarios could potentially create a binding agreement that a court would enforce, even without a formal contract document in sight.
For contract managers and legal teams, determining exactly when these exchanges cross into binding contract territory isn’t just a theoretical exercise—it’s essential to protecting company interests and preventing unexpected obligations. The stakes are high: an agreement mistakenly thought to be exploratory could suddenly become enforceable, while a supposedly solid deal might collapse due to missing elements.
This article offers practical guidance for professionals looking to strengthen their approach to creating truly enforceable contracts. Whether you’re overseeing thousands of agreements for a global enterprise or seeking to improve contract processes for a growing business, these fundamentals will help you build more secure contractual relationships while reducing legal risk.
Understanding Binding Contracts: What Does “Legally Binding” Really Mean?
Let’s start with the basics. What does legally binding mean? A binding contract is an agreement that can be enforced by law. It creates legal obligations that parties must fulfill, with potential legal consequences if they fail to do so.
The concept seems straightforward, yet in practice, determining whether an agreement truly qualifies as a binding contract, meaning enforceable in court, can be trickier than it would seem. Courts don’t just look at whether documents are labeled “contract”; they examine the substance of the agreement and the behaviors of all parties involved.
For contract managers, this means thinking through the actions taken by both sides. An exchange of emails can potentially create binding obligations. A verbal agreement during a business lunch might be enforceable. Even a handshake deal can constitute a valid contract under the right circumstances.
The Six Essential Elements of an Enforceable Contract
For a contract to be considered a truly enforceable contract, legal systems across most jurisdictions require six fundamental elements:
- Offer: One party must make a clear proposal to another, specifying what they will provide or what they expect.
- Acceptance: The other party must clearly agree to the terms of the offer without introducing new conditions.
- Consideration: Both parties must exchange something of value: money, goods, services, or even a promise to do (or not do) something.
- Intention: All parties must intend to create a legally binding relationship, not just make a casual promise.
- Capacity: The involved parties must have the legal ability to enter into contracts (related to age, mental state, and authority).
- Legality: The subject matter of the contract must be legal and not against public policy.
When all these elements are in place, you have a contract that courts will recognize and enforce. Missing any single element can render an agreement unenforceable, regardless of how formal the document appears, or if you sign a contract, is it legally binding in appearance.
When Informal Agreements Become Binding Contracts
A challenging scenario arises in determining at what point an informal agreement becomes a binding contract. Business relationships often begin with informal discussions, progress through preliminary agreements, and eventually culminate in a formal contract.
Courts typically apply an objective test: Would a reasonable person, looking at all the communications and behaviors involved, conclude that the parties intended to create legal obligations? Factors considered include:
- The language used in communications
- Whether key terms have been agreed upon
- If parties have started performing their obligations
- Previous business practices between the parties
- Documentation that followed verbal discussions
This is where many organizations encounter trouble—what executives consider “just exploring possibilities” might legally constitute a binding agreement definition that creates enforceable obligations. Even emails saying “we’re good to go” following term discussions could potentially create binding commitments.
Non-Binding Agreements: Purpose and Applications
Not every business document is meant to create immediate legal obligations. Non-binding contract documents serve important functions in the business relationship development process. These preliminary agreements help parties explore potential relationships, document progress in negotiations, and establish frameworks for future binding contracts.
Organizations intentionally use non-binding language when they want to document their actions without committing to a binding agreement just yet. This approach is both practical and pragmatic as relationships between parties often need time to develop while both parties further determine commercial terms.
Simply labeling a document “non-binding” isn’t a guarantee of it not being legally binding. Courts look to the substance of the document and not just at labels. If parties begin acting as though an agreement is binding, courts will view that as pointing to a binding agreement being present.
Common Types of Non-Binding Contract Documents
Several document types are typically intended to be non-binding, though each requires careful drafting to maintain this status:
- Letters of Intent (LOIs): Documents outlining proposed terms for future agreements, often used in business acquisitions or major deals.
- Memoranda of Understanding (MOUs): Documents capturing points of agreement in principle while acknowledging that binding obligations will come later.
- Term Sheets: Documents summarizing key points of proposed business relationships, common in investment and financing contexts.
- Statements of Work (SOWs): Documents stipulating specific project requirements and milestones that may precede binding service agreements.
For these documents to remain truly non-binding, they should explicitly state their non-binding nature, identify specific contingencies that must be met before binding obligations arise, and avoid language suggesting immediate commitment.
Binding vs Non-Binding: Strategic Considerations for Contract Managers
Contract managers face important strategic decisions when determining whether agreements should be binding vs non-binding. These decisions balance business needs for flexibility against the desire for certainty and enforceability.
Choosing binding agreements makes sense when:
- Core business terms are fully negotiated and agreed upon
- Both parties are ready to commit resources
- The arrangement requires immediate legal protection
- Risk allocation between parties is clearly defined
Opting for non-binding approaches is appropriate when:
- Due diligence is still underway
- Key terms remain under negotiation
- Stakeholder or regulatory approvals are pending
- Parties need a framework to guide complex negotiations
Understanding this distinction helps contract professionals structure agreements that protect organizational interests while supporting business relationships through various stages of development.
The Path to Enforceability: How to Make a Contract Legally Binding
Creating contracts that stand up to legal challenges requires attention to both substance and process. How to make a contract legally binding involves more than adding signature blocks and formal language. It requires deliberate attention to the essential elements previously discussed.
Successful contract managers follow specific steps to ensure the final contracts reflect both parties’ intentions and ensure legal enforceability with the support of their legal team. This begins with precisely and specifically defining all terms, including but not limited to performance obligations, payment terms, delivery expectations, and risk allocation. Ambiguity becomes the enemy of enforcement, so clarity must be the priority in drafting.
Documentation of the contract formation process also matters significantly. Maintaining records of all substantive discussions, term negotiations, and revisions creates an evidence trail that can prove invaluable if disputes arise. This documentation helps demonstrate that all parties understood what they were agreeing to—a critical factor in enforceability.
Digital Transformation: E-Signatures and Binding Agreements
The question of whether electronic agreements create binding contracts has been definitively answered: yes, they can. With the adoption of laws like the Electronic Signatures in Global and National Commerce Act (E-Sign Act) and the Uniform Electronic Transactions Act (UETA) in the US, and similar laws globally, electronic signatures now carry essentially the same legal weight as handwritten signatures.
For an electronic signature to create a binding agreement, it must generally demonstrate:
- Intent to sign: The signer intended to apply their signature to the document.
- Consent to do business electronically: Parties agreed to conduct transactions digitally.
- Clear attribution: The signature can be credibly linked to the specific individual.
- Record preservation: The signed document is stored in a way that preserves its integrity.
Modern CLM platforms incorporate these requirements into their e-signature workflows, creating legally defensible signing processes that satisfy regulatory requirements across multiple jurisdictions.
Binding Contracts Examples: Learning from Common Scenarios
Binding contracts examples appear in virtually every aspect of business operations. Understanding how binding principles apply in different contexts helps contract managers identify enforceability issues across their contract portfolios.
Consider these scenarios:
- Supply Agreements: When a supplier confirms specific pricing and delivery terms by email and the buyer responds with “Confirmed, please proceed,” this exchange likely creates binding obligations even without a formal contract document.
- Service Engagements: If a client approves a proposal that contains specific deliverables, timelines, and payment terms, work authorization may create binding commitments even before final contracts are signed.
- Employment Relationships: Offer letters containing compensation details, start dates, and role descriptions can create binding employment contracts when accepted, even if more detailed agreements follow later.
Through these examples, one can see how binding agreement principles apply across diverse business relationships, often in ways that surprise those without legal training.
Common Pitfalls That Undermine Contract Enforceability
Even carefully drafted contracts can fail the enforceability test if they contain certain flaws. Contract managers should be vigilant about these common issues that can render agreements unenforceable:
- Incomplete agreement on essential terms: When parties leave critical elements like price, quantity, or timing for future agreement, courts may find there was never a complete meeting of the minds.
- Lack of authority: If a signatory lacks proper authorization to bind their organization, the contract may be unenforceable against that party.
- Inadequate consideration: If one party doesn’t provide anything of value in the exchange, courts may find the contract unenforceable.
- Unconscionable terms: Grossly unfair provisions that shock the conscience of the court may render entire agreements—or specific clauses—unenforceable.
- Violation of laws or public policy: Contracts that require illegal actions or violate statutory requirements will not be enforced.
These issues often emerge in high-pressure business situations when contracts are rushed or templates are used without proper customization. Implementing systematic review processes helps identify these problems before they undermine important agreements.
Creating Clear Binding Agreement Definitions
Creating clarity in a binding agreement requires attention to language precision. Ambiguous wording stands as a frequent cause of contract disputes and enforcement challenges across all industries. The contract manager who masters clarity dramatically improves contract enforceability and reduces litigation risk.
Clear definitions begin with the elimination of technical jargon or, when industry terminology must be used, explicit definitions of those specialized terms. Performance requirements demand particular attention—vague phrases like “best efforts” or “industry standard quality” invite disagreement, while specific, measurable outcomes create certainty.
All conditions preceding obligations should be unmistakably defined, as should the exact timelines governing performance. Perhaps most critically, the contract should articulate what specific circumstances constitute a breach, removing any guesswork about when remedies become available.
This level of precision doesn’t emerge naturally. It requires thoughtful collaboration between business stakeholders who understand operational realities and legal professionals who comprehend enforceability requirements. When these perspectives merge successfully, the resulting contract language creates a binding agreement definition that leaves little room for interpretation or dispute.
Leveraging CLM Technology for Stronger Binding Agreements
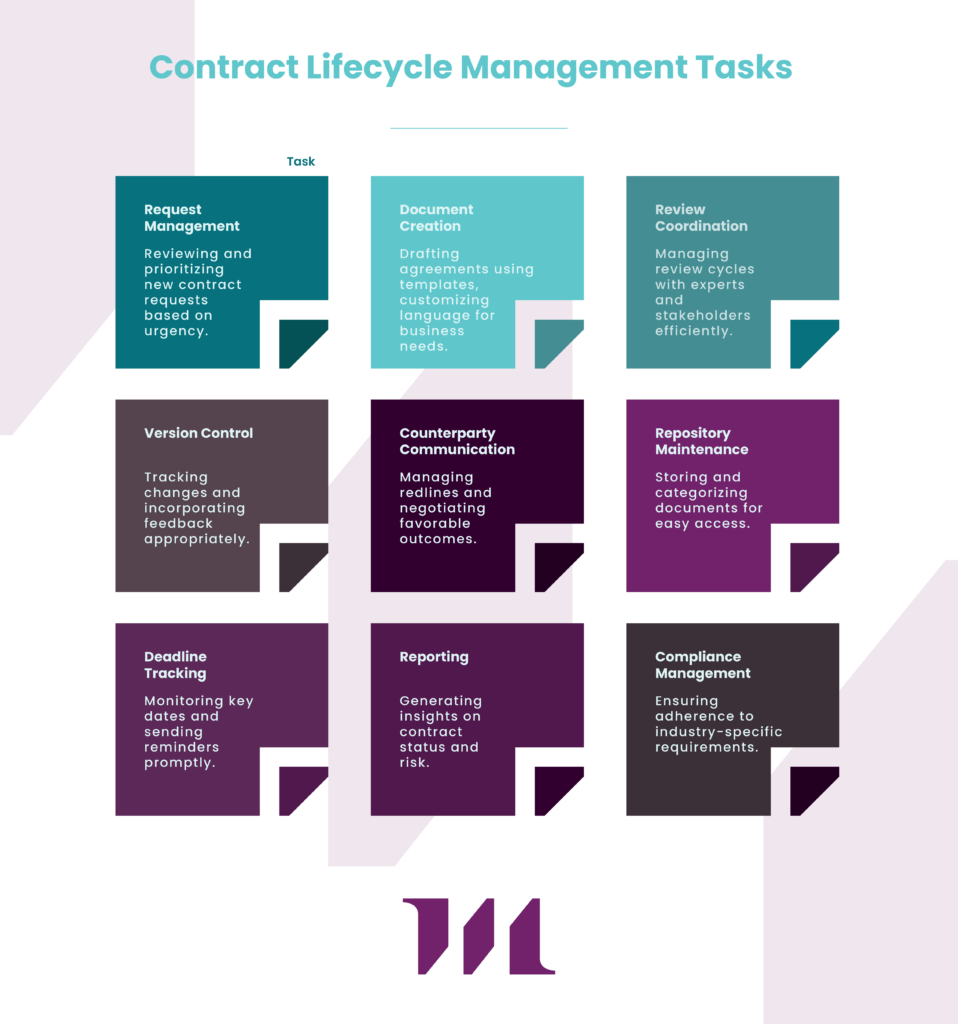
Contract Lifecycle Management systems transform how organizations create and manage binding contracts. These platforms provide structured processes for contract creation, approval, execution, and management, significantly reducing the risk of enforceability problems.
CLM technology addresses common contract challenges through:
- Template standardization: Using pre-approved language that incorporates all enforceability requirements
- Consistent clause libraries: Maintaining legally vetted language for common provisions
- Approval workflows: Ensuring proper review by all stakeholders
- Execution tracking: Documenting the signature process completely
- Obligation management: Monitoring performance against contractual requirements
For organizations managing hundreds or thousands of agreements, these capabilities dramatically reduce the risk of inadvertently creating unenforceable agreements or missing critical terms in binding contracts.
AI-Powered Contract Review: Ensuring Enforceability at Scale
Advanced CLM platforms like Malbek now incorporate artificial intelligence capabilities that specifically address enforceability concerns across large contract portfolios. These systems analyze contract language to identify potential issues before they become problems.
Malbek’s AI-powered contract review features exemplify this approach. The platform’s Ensemble LLM approach—which dynamically selects appropriate language models for specific use cases—helps contract managers spot problematic language, missing terms, and inconsistencies that could undermine enforceability.
This technology systematically examines agreements against established legal standards, flagging potential issues like:
- Missing essential contract elements
- Ambiguous performance obligations
- Contradictory provisions
- Unusual or non-standard terms
- Potentially unenforceable clauses
For contract managers balancing heavy workloads, this capability acts as a virtual legal assistant, applying enforcement principles consistently across all agreements.
Automating the Journey from Non-Binding to Binding Agreements
The progression from initial business discussions to final binding contracts rarely happens overnight. CLM systems recognize this reality by supporting and facilitating the complete contracting process from preliminary non-binding discussions through final execution.
Malbek’s platform illustrates how this works in practice. The system manages the drafting and exchange of preliminary non-binding documents like LOIs while maintaining a clear distinction between these exploratory documents and final binding agreements. As negotiations progress, the system tracks revisions and maintains version control, creating a comprehensive audit trail that documents exactly when and how parties reached final agreement.
This approach helps organizations maintain appropriate distinctions between binding vs non-binding documents while facilitating the smooth progression from initial discussions to enforceable agreements.
Conclusion
Understanding the distinction between binding and non-binding contracts is fundamental to effective contract management. The difference affects everything from negotiation strategies to risk management approaches and influences how organizations structure their contract processes.
For contract managers and legal professionals, leveraging CLM technology has become essential to managing these distinctions at scale. By implementing systematic approaches to contract creation, review, and management, organizations can ensure their agreements achieve the intended legal effect, whether that’s creating immediate binding obligations or establishing frameworks for future relationships.
The ultimate goal remains consistent: creating clear, enforceable agreements that protect organizational interests while supporting productive business relationships. With the right combination of legal knowledge and technological support, today’s contract professionals can achieve this balance across even the most complex contract portfolios. Discover how Malbek’s AI-powered CLM platform can transform your approach to creating and managing binding contracts by requesting a demonstration today. Experience firsthand how Malbek’s unique Ensemble LLM approach and intuitive interface can help your team reduce risk while accelerating the contract lifecycle from initial discussions to final execution.